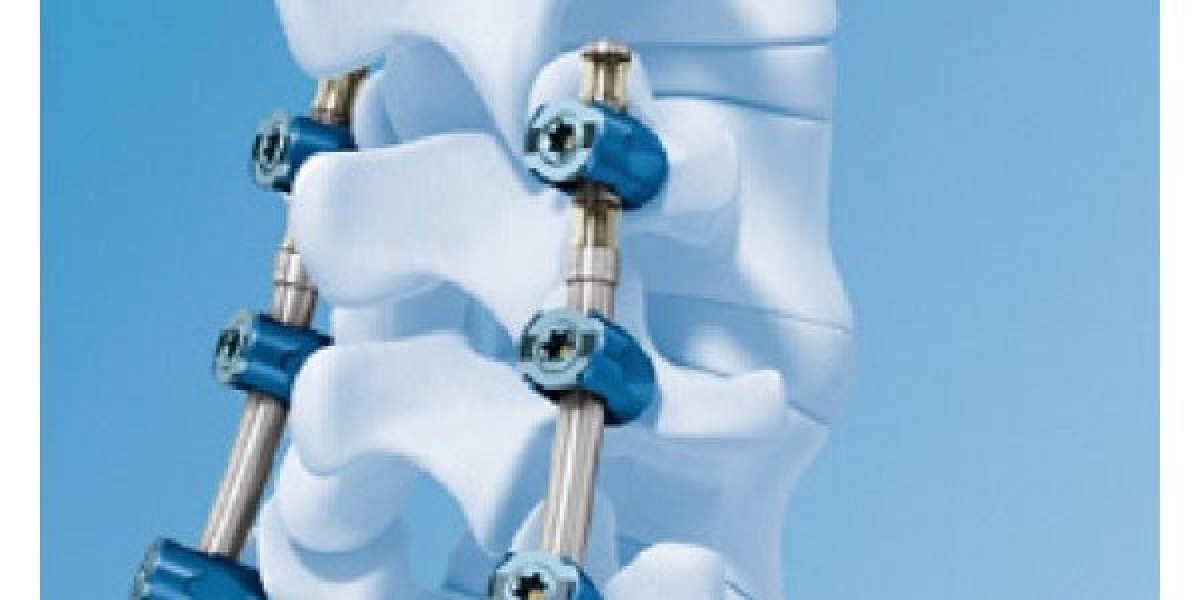
Early Development and Testing of Artificial Discs Technology
The technology of fabricated discs emerged in the 1980s as a small number of surgeons and engineers began experimenting with silicone and metal designs to potentially provide an alternative to spinal fusion surgery. Initial artificial disc designs were tested in animals and cadavers to evaluate biocompatibility and mechanical function. By the 1990s, early prototypes made of metal and plastic composites were implanted in limited human clinical trials to assess safety and potential advantages over fusion. These early trials produced encouraging results and helped identify design improvements needed to better replicate the complex biomechanics of natural spinal discs.
Clinical Trials in Europe Lead to Regulatory Approvals
As Artificial Discs design and implantation techniques advanced through the 1990s, larger-scale clinical trials involving hundreds of patients were initiated across Europe. Trial results published in leading medical journals compared artificial disc replacement to fusion for specific spinal levels and diagnoses. The trials consistently reported superior outcomes related to pain relief and mobility for disc replacement compared to fusion. Based on the positive and growing body of evidence, artificial disc devices received regulatory approval in Europe starting in the late 1990s, allowing their broader adoption in routine clinical use on that continent. European spine surgeons quickly incorporated fabricated discs into their repertoire, performing many thousands of procedures annually.
FDA Approval and Introduction to the US Marketplace
While enjoying widespread adoption in Europe, artificial disc technology faced more regulatory hurdles for introduction in the large US marketplace. Extensive pre-clinical testing and multi-year clinical trials involving over 500 patients were required by the FDA before approval could be granted. Finally, in 2006-2007, two artificial disc designs received PMA approval from the FDA, paving the way for their use in spine practices across America. Initial uptake was predictably slow as surgeons gained experience with the new category of implants. However, ongoing published evidence indicated outcomes were sustaining or improving as compared to fusion. By 2010, fabricated discs had become an established treatment option for indicated spinal conditions in major medical centers nationwide.
Outcome Evidence Compares Favorably to Fusion
Perhaps the most compelling evidence supporting the role of fabricated discs comes from long-term outcome comparisons to fusion surgery. Multiple studies have now reported 5-10 year follow-up on large patient cohorts. These studies consistently demonstrate fabricated discs providing equivalent or better pain relief when compared to fusion, often with superior functionality and fewer activity limitations. Additionally, the risk of adjacent level complications is significantly reduced compared to fusion, which has led post-fusion problems over the long term for many patients. Perhaps most importantly, fabricated discs preserve spinal motion and avoid accelerated degenerative changes that commonly occur above and below fused spinal segments. This motion preservation effect is believed responsible for the more durable and longer lasting clinical benefits of disc replacement relative to fusion.
Post-Approval Improvements Expand Indications
Since gaining initial approval, implanted artificial disc designs have continued to evolve based on growing implantation experience and focus on improving longevity, reducing risks of complications, and expanding the eligible patient population. Material science and mechanical engineering enhancements have provided discs with higher strength, endurance, and finer angular movement capabilities replicating healthy disc biomechanics. Added imaging and navigation technologies now assist surgeons in precise implantation. Revision procedures for post-deployment issues or limited movement have achieved good outcomes. Collectively, these improvements have allowed extension of artificial disc usage to more spinal levels and additional diagnoses beyond the initial lumbarindications. Younger and more active patients are increasingly offered disc replacement where once only fusion might have been recommended. The broader experience suggests fabricated discs may serve as durable long-term solutions in appropriately indicated cases.
over three decades, artificial discs technology has graduated from initial concepts to becoming a proven and established surgery for specified spinal conditions. Where once spinal fusion was often the sole option, disc replacement has emerged as a clinically superior alternative for preserving spinal mobility and maximizing long-term functional outcomes for patients. Ongoing enhancements continue broadening the appropriate usage of these implants. Fabricated discs reflect the promise of joint replacement orthopedics to replace otherwise mechanical and less physiological fusions. The history and accrued evidence validate fabricated discs as effective treatments that can relieve back/leg pain while allowing patients to resume active lifestyles without risk of adjacent level degradation common after fusion. For indicated cases, fabricated discs represent a milestone in progressing spine care beyond fusion towards restored natural motion.
Get More Insights On Artificial Discs
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement.
(LinkedIn- https://www.linkedin.com/in/priya-pandey-8417a8173/)