The combination product services market is anticipated to grow at a CAGR of around 10%, till 2035, claims Roots Analysis
Growing interest for combination products, coupled with the ongoing challenges of combining drug / biologic with a medical device, is anticipated to drive the demand for outsourcing combination products development and testing.
Roots Analysis has announced the addition of “Combination Product Services Market, 2022-2035” report to its list of offerings.
The development of drug-device combination products is a long-drawn-out process that not only requires novel technologies and processes but also a huge amount of capital and expertise. In order to overcome these challenges and enhance overall patient experience, several developers are outsourcing their operations to companies that offer services for testing and validation of combination products.
Key Market Insights
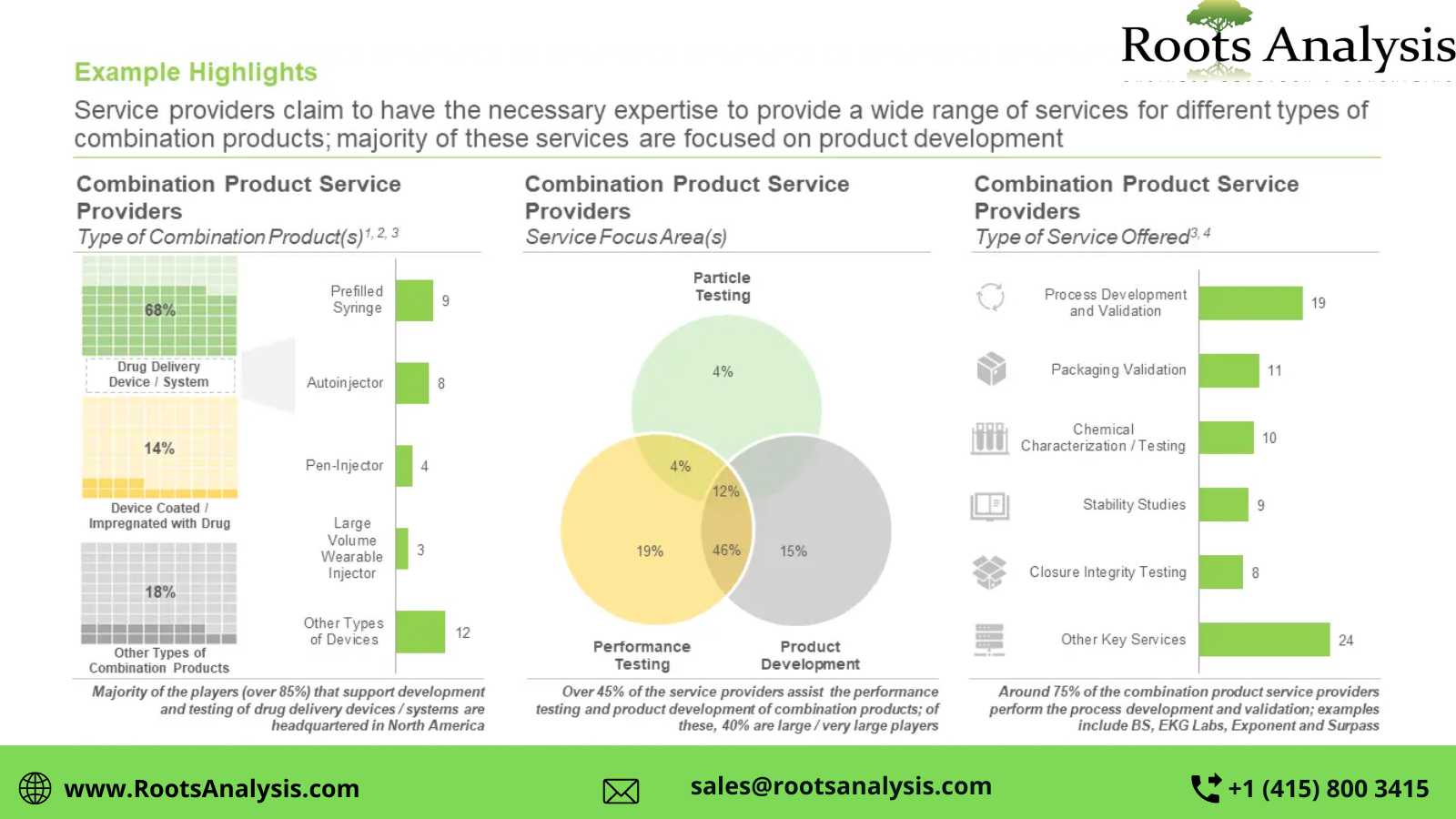
Currently, more than 25 service providers claim to offer services for combination products
The market is dominated by the presence of small (11-50 employees) and mid-sized (51-500 employees) players, who represent over 55% of the contemporary market landscape. Additionally, it is worth noting that majority (81%) of the service providers are based in North America.
Around 70% of the players engaged in this industry offer services for drug delivery device / system
Over 65% of the aforementioned players offer services for product development. Further, 60% of the combination product service providers offer services for prefilled syringes. Notably, over 70% of the total players engaged in this space also offer process development and validation services.
Go / No-Go framework can be used to assist players in the outsourcing decision-making process
The framework enables evaluation of the current capabilities of prefilled syringe combination product developers and aids them to take the crucial decision of outsourcing / in-house development and testing of prefilled syringe combination products based on 5+ parameters.
Expansion activity within this field has grown significantly between 2017 and 2022
Maximum number of expansion initiatives (38%) were undertaken in 2021. It is worth highlighting that majority of the expansions were facility expansions. Notably, over 70% of the expansion initiatives have been undertaken for facilities based in North America.
North America and Europe are anticipated to capture larger share (over 65%) of market by 2035
Growth in this domain is anticipated to be primarily driven by the rising demand of combination products. Players providing performance testing services are anticipated to capture more than 75% of the market by 2035.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/....reports/combination-
The financial opportunity within the Combination Product Services (Focus on Particle Testing, Performance Testing and Product Development) Market has been analyzed across the following segments:
Type of Device
Service Focus Area
Company Size
Geographical Regions
For additional details, please visit https://www.rootsanalysis.com/....reports/combination- or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Medical Devices Contract Research Organization Market (3rd Edition), 2022-2035
2. Prefilled Syringes Market (6th Edition), 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com